Get Healthy!
- Robert Preidt and Robin Foster
- Posted October 28, 2021
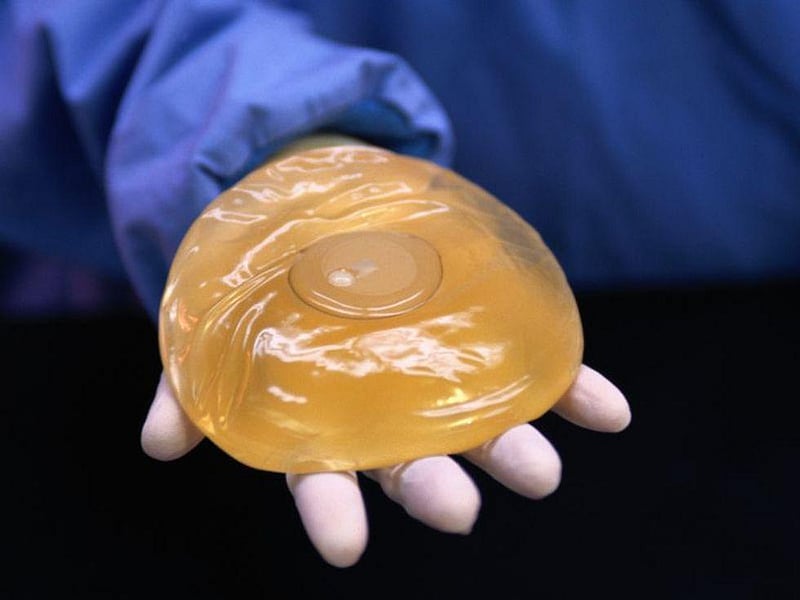
Stronger Breast Implant Safety Measures Announced by FDA
A boxed warning and a checklist of risks that must be shared with patients is among the new breast implant safety measures announced by the U.S. Food and Drug Administration on Wednesday.
As the FDA "continues to evaluate the overall effects of breast implants in patients, today's actions help ensure that all patients receive the information they need to make well-informed decisions affecting their long-term, personal health," Dr. Binita Ashar, director of the FDA's Office of Surgical and Infection Control Devices, said in an agency news release. "Protecting patients' health when they are treated with a medical device is our most important priority."
Only health care providers and facilities that provide the checklist will be allowed to sell and distribute breast implants, the agency noted.
The checklist "must be reviewed with the prospective patient by the health care provider to help ensure the patient understands the risks, benefits and other information about the breast implant device," and the patient must have the opportunity to sign the checklist, which also has to be signed by the physician implanting the device, the FDA added.
The agency also updated recommendations on screening patients for ruptures in silicone gel-filled breast implants.
Manufacturers have 30 days to post the updated labeling on their websites, the FDA said.
It also released updated information on the status of FDA-mandated studies by breast implant manufacturers after their devices were approved by the agency.
Tens of thousands of women who received breast implants later developed brain fog, fatigue and other health issues collectively referred to as "breast implant illness," and activists have long pushed for an informed consent process so patients clearly understand the risks and benefits of implants before receiving them, the Washington Post reported.
More information
Visit the U.S. Food and Drug Administration for more on breast implants.
